EXCERPTED FROM: University of Manchester, Department of Chemistry
Credit to: T. WallaceConformations of butane
The conformational possibilities increase as alkanes become larger. A plot of potential energy against rotation about the C(2)–C(3) bond in butane is shown below. The lowest-energy arrangement, called the antiperiplanar (or anti) conformation, is the one in which the two large methyl groups are as far apart as possible. As rotation around the C(2)–C(3) bond occurs, another eclipsed conformation (anticlinal) is reached in which there are two Me–H interactions and one H–H interaction. If we assign the energy value (4 kJ/mol) for H–H eclipsing interactions that was previously derived from ethane, we can predict that each Me–H interaction in the anticlinal conformation costs about 5 kJ/mol.
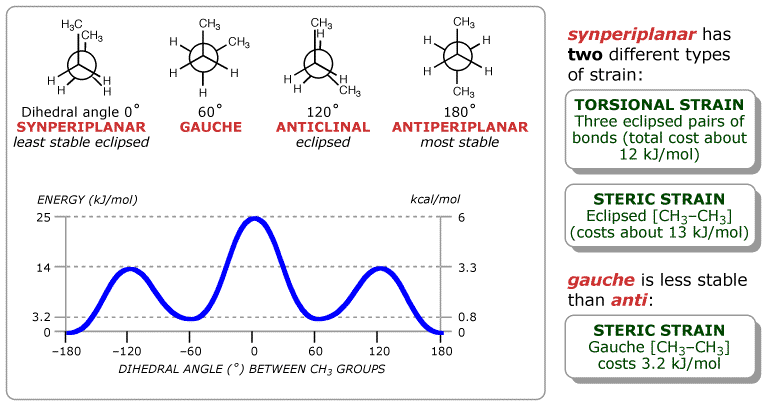
As bond rotation continues, an energy minimum is reached at the staggered conformation where the methyl groups are 60° apart (a gauche relationship). This lies 3.2 kJ/mol higher in energy than the anti conformation even though it has no eclipsing interactions. This energy difference is due to the fact that the hydrogen atoms of the methyl groups are near each other in the gauche conformation, resulting in steric strain, which is the repulsive interaction that occurs when atoms would otherwise tend to occupy the same space.
As the dihedral angle between the methyl groups approaches 0°, an energy maximum is reached. The methyl groups are forced even closer together than in the gauche conformation, and both torsional strain and steric strain are present. A total strain energy of 25 kJ/mol has been estimated for this conformation, allowing us to calculate a value of 17 kJ/mol for the Me–Me eclipsing interaction.
Completing the 360° rotation after the synperiplanar point produces the mirror images of what we've already seen; another gauche conformation, another eclipsed conformation and finally a return to the anti conformation.Use the interactive model below to explore the conformations of butane. In particular, try the following 'visualisation' of steric strain: Select Reset, Atoms 100%, coloured methyls and Animate loop, then watch the interactions of the two methyl groups as they pass by each other. The butane structure used in this animation was generated by quantum chemical calculation using the 6-31G* basis set.
|
|
CONTROLS new resizable window |
|
Go to conformations of ethane |