EXCERPTED FROM: University of Manchester, Department of Chemistry
Credit to: T. WallaceConformations of ethane
Experiments show that there is a 12 kJ/mol (2.9 kcal/mol) barrier to rotation in ethane. The most stable (low energy) conformation is the one in which all six C–H bonds are as far away from each other as possible (staggered when viewed end-on in a Newman projection). The least stable (high energy) conformation is the one in which the six carbon–hydrogen bonds are as close as possible (eclipsed in a Newman projection). All other conformations lie between these two limits. The barrier to rotation is the result of three equal C–H bond-eclipsing interactions, so we can assign a value of about 4.0 kJ/mol (1.0 kcal/mol) to each of these interactions. The corresponding energy in propane is 14 kJ/mol (3.4 kcal/mol).
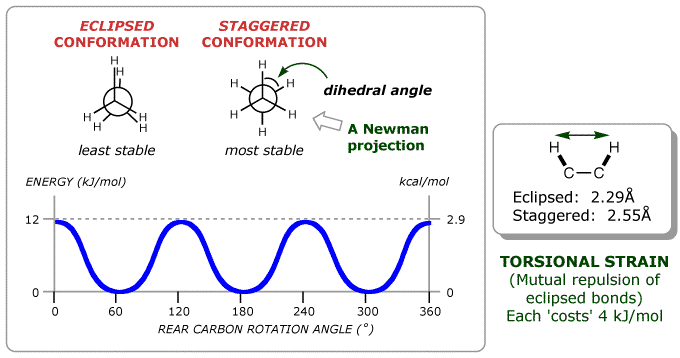
The 12 kJ/mol of extra energy in the eclipsed conformation of ethane is called torsional strain. The barrier to rotation that results from this strain can be represented in a graph of potential energy versus degree of rotation in which the angle between C–H bonds on C-1 and C-2 (the dihedral angle) completes one revolution. Energy minima occur at staggered conformations, and energy maxima occur at eclipsed conformations. The torsional strain is thought to be due to the slight repulsion between electron clouds in the eclipsed bonds.
We can represent conformational isomers in one of two ways. Sawhorse representations view the carbon–carbon bond at an angle so as to show the spatial orientation of all C–H bonds. In a Newman projection the carbon–carbon bond is viewed along its axis and the two carbon atoms are repesented by a circle. The bonds attached to the front carbon are represented by lines going to the centre of the circle, and bonds attached to the rear carbon are represented by lines going to the edge of the circle. The advantages of Newman projections are that they are easy to draw and they clearly show the relationships among substituents on the different carbon atoms.
Use the interactive model below to explore the conformations of ethane.
|
|
CONTROLS new resizable window |
|
Go to conformations of propane |